| Adverse Reaction1* (≥10%) in patients taking AUGTYRO |
AUGTYRO (N=426) | |
|---|---|---|
| ALL GRADES % | GRADE 3 OR 4 % | |
| Nervous System Disorders | ||
| Dizzinessa | 65 | 2.8 |
| Dysgeusiab | 54 | 0 |
| Peripheral neuropathyc | 49 | 1.4 |
| Ataxiad | 28 | 0.5 |
| Cognitive impairmente | 25 | 0.9 |
| Headachef | 19 | 0 |
| Gastrointestinal Disorders | ||
| Constipation | 38 | 0.2 |
| Nausea | 20 | 0.7 |
| Diarrhea | 14 | 0.7 |
| Vomiting | 12 | 1.2 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||
| Dyspneag | 30 | 6 |
| Coughh | 18 | 0.2 |
| Pneumoniai | 11 | 6 |
| General Disorders | ||
| Fatiguej | 30 | 1.2 |
| Edemak | 15 | 0.5 |
| Decreased appetite | 11 | 0.2 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Muscular weakness | 20 | 2 |
| Myalgial | 13 | 0.7 |
| Metabolism and Nutritional | ||
| Increased weight | 16 | 3 |
| Eye Disorders | ||
| Vision disordersm | 12 | 0.5 |
Discover a path forward with AUGTYRO (repotrectinib)
A next-generation TKI for ROS1+ NSCLC, approved for NTRK gene fusion+ solid tumors1,2
INDICATION
This indication is approved under accelerated approval based on overall response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in the confirmatory trial(s). |
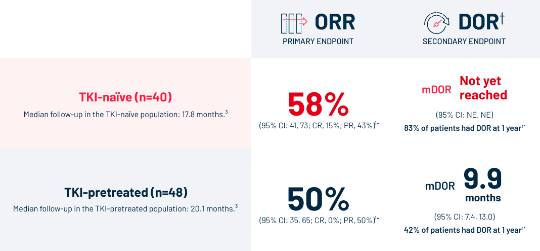
EFFICACY
ORR and DOR in TKI-naïve and ‑pretreated patients1*
Data from the pivotal TRIDENT-1 study*
Intracranial response
SECONDARY ENDPOINT
 icORR
icORR
SECONDARY ENDPOINT
in patients with measurable CNS metastases at baseline1*‡
2/2
TKI‑naïve patients
achieved intracranial response
3/3
TKI-pretreated patients
achieved intracranial response
| * | Data from the pivotal TRIDENT-1 study, a Phase 1/2 multicenter, single-arm, open-label, multicohort clinical trial of AUGTYRO (160 mg orally once daily for 14 days, then increased to 160 mg twice daily until disease progression or unacceptable toxicity) in adult patients with locally advanced or metastatic NTRK gene fusion+ solid tumors. The major efficacy outcome measures were ORR and DOR according to RECIST v1.1 as assessed by BICR. Intracranial response according to modified RECIST v1.1 was assessed by BICR. The primary efficacy populations included 40 TRK TKI-naïve patients and 48 patients who received prior TRK TKI. Inclusion criteria: NTRK gene fusion+ (NTRK1/2/3) locally advanced or metastatic solid tumors; ECOG performance status ≤1; measurable disease per RECIST v.1.1. Key exclusion criteria: patients with symptomatic brain metastases.1 |
| † | Median DOR (95% CI) are based on Kaplan-Meier estimates. DOR results are based on the updated data as of 15 October 2023. DOR landmark analysis is based on the observed DOR.1 |
| ‡ | One out of 2 TKI-naïve patients and 2 out of 3 TKI-pretreated patients received prior radiotherapy to the brain, all more than 2 months prior to study entry.1 |
SAFETY
Adverse reaction profile with AUGTYRO in the TRIDENT-1 study1
The safety population included 426 patients who were exposed to AUGTYRO1

Serious adverse reactions occurred in 35% of patients who received AUGTYRO. Serious adverse reactions in ≥2% of patients included pneumonia, dyspnea, pleural effusion, and hypoxia. Fatal adverse reactions occurred in 3.5% of patients who received AUGTYRO, including pneumonia, pneumonia aspiration, cardiac arrest, sudden cardiac death, cardiac failure, hypoxia, dyspnea, respiratory failure, tremor, and disseminated intravascular coagulation.
| * | Based on NCI CTCAE v4.03. |
| a | Includes terms dizziness, vertigo, dizziness postural, dizziness exertional, vertigo positional. |
| b | Includes terms dysgeusia, ageusia, anosmia, hypogeusia. |
| c | Includes terms neuralgia, neuropathy peripheral, peripheral sensory neuropathy, dysesthesia, peripheral motor neuropathy, polyneuropathy, paresthesia, hypoesthesia, hyperesthesia. |
| d | Includes terms ataxia, gait disturbance, balance disorder, cerebellar ataxia and coordination abnormal. |
| e | Includes terms memory impairment, disturbance in attention, cognitive disorder, confusional state, amnesia, attention deficit hyperactivity disorder, delirium, altered state of consciousness, aphasia, delusion, depressed level of consciousness, hallucination, mental status changes, neurological decompensation. |
| f | Includes terms headache, migraine, tension headache. |
| g | Includes terms dyspnea and dyspnea exertional. |
| h | Includes terms productive cough, cough, and upper-airway cough syndrome. |
| i | Includes terms pneumonia, pneumonia aspiration, lower respiratory tract infection, pneumonia viral, pneumonia bacterial, lower respiratory tract infection bacterial, pneumonia klebsiella. |
| j | Includes terms fatigue and asthenia. |
| k | Includes terms generalized edema, periorbital edema, localized edema, face edema, edema peripheral, edema, eye edema, scrotal edema. |
| l | Includes terms myalgia, myositis, musculoskeletal discomfort, musculoskeletal pain. |
| m | Includes terms vision blurred, dry eye, visual impairment, visual field defect, cataract, conjunctivitis, eye pain, photophobia, photosensitivity reaction, visual acuity reduced, vitreous floaters, blepharospasm, color blindness, diplopia, eye hematoma, eye swelling, eyelid disorder, eyelid injury, eyelids pruritus, glaucoma, night blindness, ophthalmic herpes zoster. |
| BICR=blinded independent central review; CNS=central nervous system; CR=complete response; CTCAE=Common Terminology Criteria for Adverse Events; DOR=duration of response; ECOG=Eastern Cooperative Oncology Group; icORR=intracranial objective response rate; mDOR=median duration of response; NCI=National Cancer Institute; NE=not evaluable, endpoint not yet reached; NSCLC=non-small cell lung cancer; NTRK=neurotrophic tyrosine receptor kinase; ORR=objective response rate; PR=partial response; RECIST=Response Evaluation Criteria In Solid Tumors; ROS1=proto-oncogene C-Ros1, receptor tyrosine kinase; TKI=tyrosine kinase inhibitor. |
References:
1. AUGTYRO [package insert]. Princeton, NJ. Bristol-Myers Squibb Company. 2. Drilon A, Ou SI, Cho BC, et al. Repotrectinib (TPX-0005) is a next-generation ROS1/TRK/ALK inhibitor that potently inhibits ROS1/TRK/ALK solvent-front mutations. Cancer Discov. 2018;8(10):1227-1236. 3. Solomon B, Drilon A, Lin JJ, et al. Repotrectinib in patients with NTRK fusion-positive advanced solid tumors, including non-small cell lung cancer: updated from the phase 1/2 TRIDENT-1 trial. Presented at: ESMO Congress 2023; October 20-24, 2023; Madrid, Spain.
